HAIR INTRODUCTION
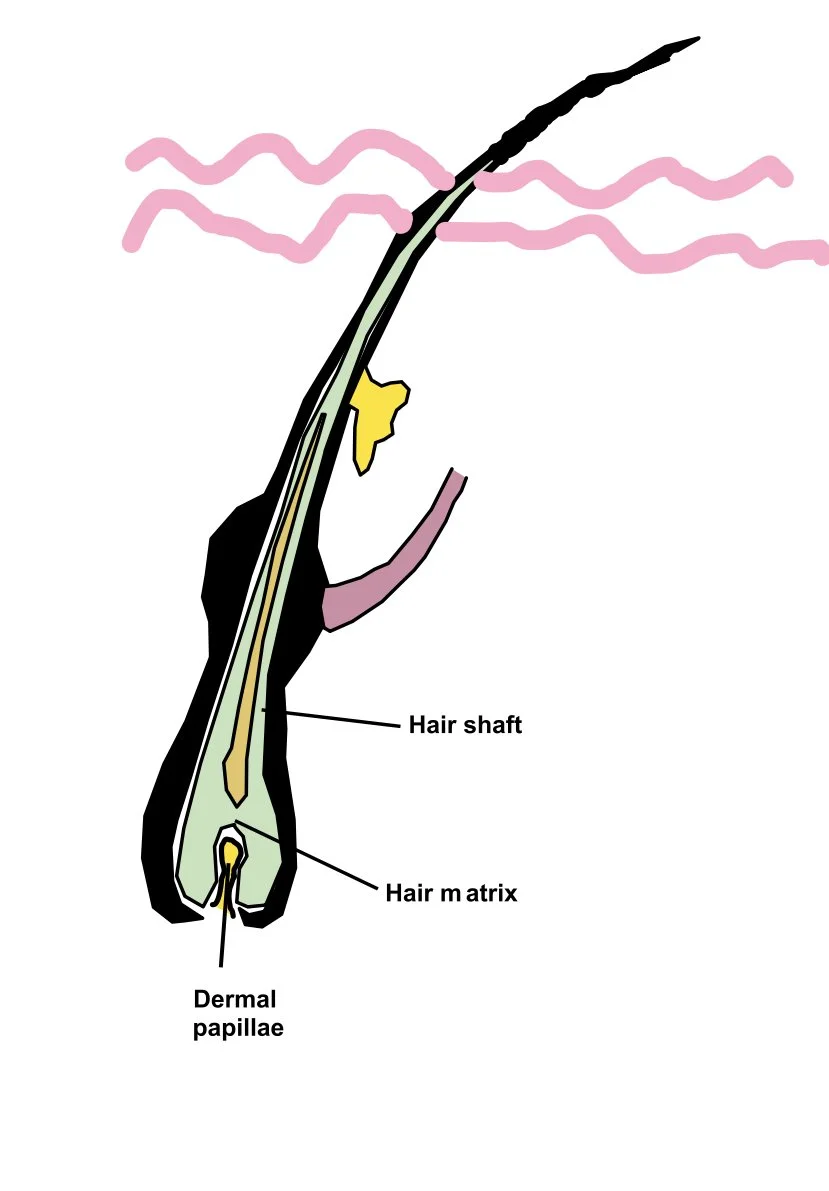
Below is a schematic of the hair follicle
Of note,
The dermal papilla is found at the base of the hair follicle. It has a rich blood supply. It contains a collection of mesenchymal cells which play a role in regulating hair growth by interacting with hair matrix cells.
The hair matrix sits above this and is contains cells responsible for producing the hair shaft. These cells divide and differentiate to from different parts of the hair shaft (eg the cuticle, cortex, medulla)
THE HAIR CYCLE
Normal human hair growth cycle occurs in distinct phases
Anagen phase (active hair with follicular growth):
In a normal scalp 90-95% of hair follicles are in the anagen phase
The matrix cells divide and produce hair cells
These differentiate and move upwards to form the hair shaft
Typically lasts 2-7 years
Catagen phase (regression with follicular involution):
< 1% hair follicles are in this phase
Is a short transition phase (2-3 weeks)
During this phase the follicle beings to shrink and detach from the dermal papilla which provides the hair follicles blood supply
Telogen phase (follicular rest and shedding of hair follicles)
5-10% hair follicles are in this phase
This is the resting phase and usually lasts about 2-4 months
The hair shaft initially remains in place but is no longer growing
Eventually the hair shaft is shed
The follicular unit then switches back into an early anagen phase where hair regrowth begins again
SCARRING VS NON SCARRING ALOPECIA
One of the main things to work out when dealing with hair loss is whether this is a scarring alopecia or a non-scarring alopecia
For instance if you get inflammation around the bulb site this results in a non-scarring alopecia (eg alopecia areata)
If you get inflammation at the bulge (around the level where the arrector pili is) then you destroy the follicular stem cells that reside in this area and you get a scarring alopecia (eg Lichen planopilaris, Discoid lupus)
IMMUNE PRIVILEGE
The final concept I would like to mention before discussing different conditions is that of immune privilege
Immune privilege is a status in which the immune system is in a state of non-reactivity to any antigens in order to prevent local tissue destructive reactions
It is charachterised by a number of mechanisms intricately working together to suppress immune attacks on cells
Sites of immune privilege include the brain, testes, anterior chamber eye
The hair follicle is a site of relative immune privilege
The mechanism in the hair follicle to keep this immune privilege include:
Production of potent immunosupressants locally (eg TGF β1, IL-10)
Decreased MHC class 1 expression on cells so that (auto) antigens on these cells can’t be shown to cytotoxic CD8 T cells
Decreased function of antigen presenting cells
A good analogy I heard at a talk at an alopecia study day with the St. John’s Institute Dermacademy was that you can think of immune privilege as like bricks around a hair follicle
You can lose these bricks at any site vertically along the hair follicle and inflammatory cells can then attack at this site
If get an inflammatory response in the bulge you get a scarring alopecia and if you get it at the bulb you get a non-scarring alopcecia
(The level the ‘brick collapse’ may occur at may be in part determined by genetics)
MAKING A DIAGNOSIS
When dealing with a case of hair loss there are a few important considerations to think about
Is it a scarring or non-scarring alopecia?
If it is a non scarring alopecia you might want to think of what location is it in?
Focal (eg alopecia areata)
Patterned - occuring in a specific pattern (eg androgenetic alopecia)
Diffuse - shedding occuring across the scalp (eg telogen effluvium, alopecia areata)
If it is scarring you can classify it based on the nature of the inflammatory infiltrate
Primarily lympmhocytic - LPP, Lupus
Primarily neutrophilic - Folliculitis decalvans
Mixed primary (eg Folliculitis decalvans/LPP overlap)
Hair workup can include:
History and exam
Trichoscopy (eg dermoscopy of scalp)
Hair pull test
+/- Biopsy
+/- Lab investigations
Hair pull test:
To evaluate excess hair shedding
Grasp about 40-60 hairs between index finger and thumb and pull gently upwards
Clasically greater than 10 hairs being removed indicates a positive test (although some consider lower amounts a positive test)
Causes of a positive pull test:
Alopecia areata
Telogen effluvium
Anagen effluvium
Lose anagen syndrome
+/- Early androgenetic alopecia
Biopsy:
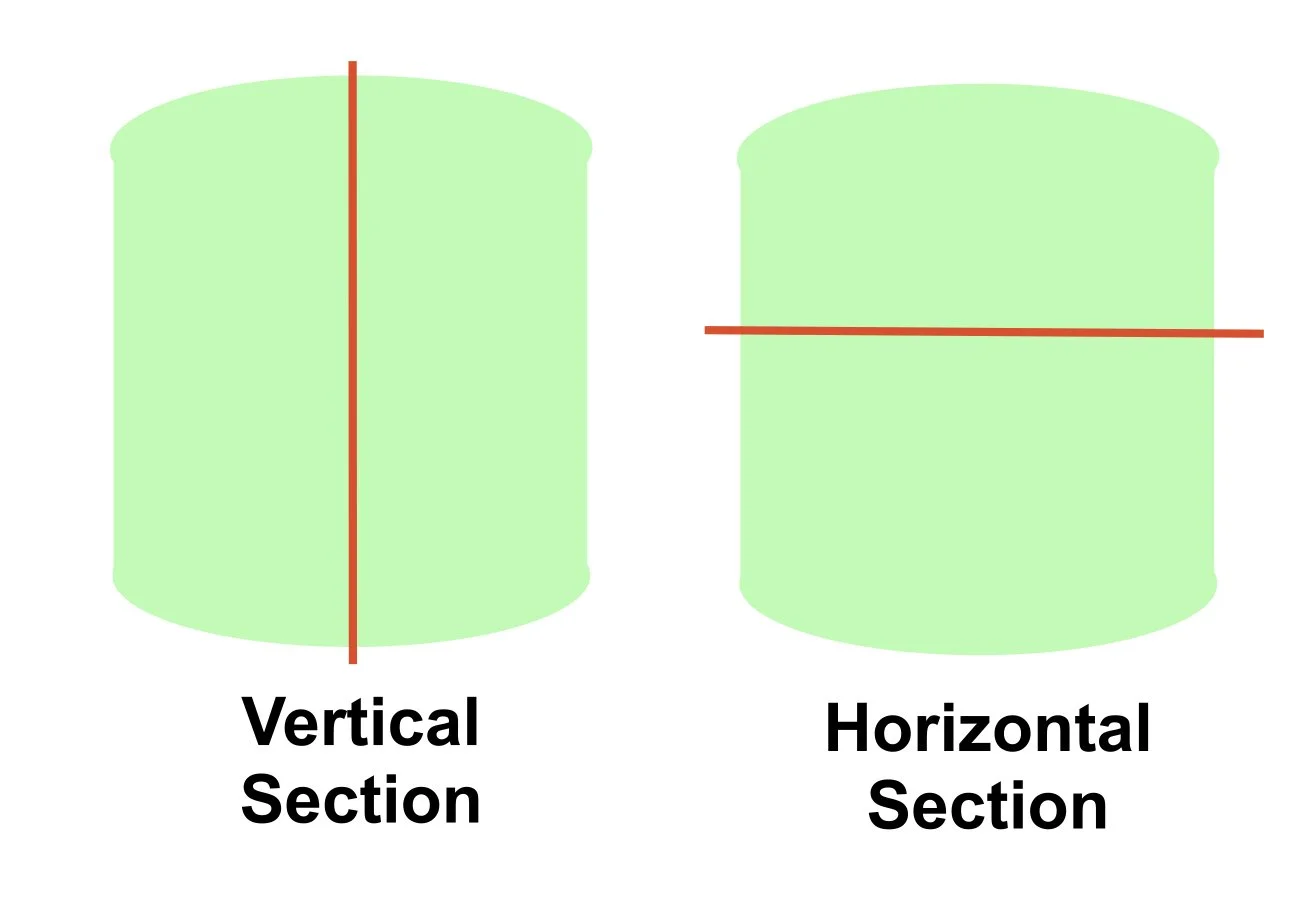
When a biopsy is performed (in particular for a scarring alopecia) you should do two punch biopsies and ask for vertical sectioning (usual type of sectioning) and horizontal sectioning
When the pathologist looks at the horizontal section it looks like they are looking down on top of the hair follicle
It allows for:
Examination of more hair follicles in one biopsy (allows examination of hair follicles at different stages of the hair cycle)
Analysis of hair follicle from top to bottom (eg from epidermis to hair bulb)
ALOPECIA AREATA
Alopecia areata is a chronic immune-mediated form of non-scarring alopecia inflammatory disease which affect the hair follicles
It is the most common immune mediated cause of hair loss worldwide
Onset may be at any age
Any hair-bearing site can be involved
It can be asscociated with autoimmune disorders and in particular atopic dermatitis, thyroid disease and less commonly SLE
Epidemiology:
Lifetime risk 1-2%
Similar rates in males and females
10-20% affected persons have family history of AA
80% of cases occur before 40 years (50% of these cases begin in childhood)
Alopecia totalis - all or nearly all of scalp hair gone
Alopecia universalis - all or nearly all hair from scalp and body gone
PATHOGENESIS
In AA there is damage to hair follicles which occur in the anagen phase leading to premature transition to the catagen and telogen phase, then back to a dystrophic anagen phase
During the active disease process, hair follicles are unable to progress past the early anagen phase
Hair follicle stem cells are spared so no hair follicles are destroyed (ie no scarring)
The pathomechansim surrounding AA is complex still not fully understood
It is multi-factorial and involves interactions between immunologic, genetic and environmental factors
Essentially in AA you get loss of follicular immune privilege and an associated T cell-mediated immune attack on cells within the hair bulb
Immune factors:
The main theory of AA pathogenesis is that it develops through the loss of immune priviliged status of hair follicles which leads to the infiltration of inflammatory cells, especially cytotoxic T cells and NK cells, into the hair bulb causing death of follicular keratinocytes
As mentioned above with immune privilege you have:
Have production of potent immunosuppressants locally
Get prevention of activation of T cells and NK cells in the area
A potential pathway in alopecia areata is that local stressors (? Trauma, infection, stress, atopy) leads to a stressed hair follicle and decreased expression of the immunosupressive cytokines IL-10 and TGF-beta (‘immune privilege guardians’)
There is now an inflammatory environment with increased pro-inflammatory cytokines such as IFN-gamma and IL-15
IFN-gamma induces expression of MHC class 1 molecules on keratinocytes making it more susceptible to recognition by cytotoxic T cells
IL-15 promotes the survival of cytotoxic T cells and NK cells
IFN-y and IL-15 singal through JAK-STAT signalling (Hence why JAK inhibitors are used in alopecia areata)
Stressed follicular keratinocytes also express more MICA which is a ligand that is normally absent or low on follicular keratinocytes (think of MICA is an alarm to signal stress)
NKG2D is a receptor that is expressed by NK cells and cytotoxic T cells that can bind to MICA
When NKG2D is activated after binding to MICA it acts as a ‘go signal’ for cytotoxic T cells and NK cells to carry out their cytotoxic functions
All the above mechanisms result in cytotoxic T cells and NK cells swarming and attacking the hair bulb causing keratinocyte apoptosis
It is important to know that it is a complicated process and pathogenesis is still not fully known other cells and cytokines that have been associated with pathogenesis include plasmacytoid dendritic cells (pDCs), IL-17, IL-23, TNFa, CCL20, CXCR3, CXCL10 among others.
Genetics:
Genetics also play a role in a patient’s susceptibility to developing alopecia areata
The HLA subtype most associated with alopecia areata are different HLA-DR subtypes and for instance HLA DRB1*11:04 allele can be associated with early onset AA and high familial recurrence risk
Other genes that have been implicated in the development of AA include:
IL2RA (Interleukin-2 receptor alpha)
CTLA4 gene
IL-10 gene
WORKUP
Clinical history and exam
Trichoscopy
Biopsy
Lab investigations
CLINICAL FEATURES
Hair loss is typically asymptomatic but can get occasional pruritus/burning prior to hair loss
Ask about the speed of onset of hair loss
Different patterns of alopecia areata can be observed
Patchy alopecia:
Commonest clinical variant
Smooth, circular, discrete areas of hair loss developing over period of a few weeks
May remain discrete or enlarge and coalesce
Oophiasis pattern:
A band-like area of alopecia extending across occipital scalp
Alopecia totalis:
Total loss of scalp hair
Alopecia universalis:
Loss of all hair over entire skin surface
Diffuse alopecia areata (aka alopecia areata incognita):
Rare form of AA predominantly seen in young women
Get rapidly progressive, generalized thinning of scalp hair
Clinical picture can resemble telogen effluvium but dermoscopic findings may point towards this diagnosis
Prognosis generally quite favourable
TRICHOSCOPY
Can be used to confirm disease and get indication of disease activity
See hair follicle orifices (absence suggests a cicatricial alopecia)
Exclamation point hairs
Common and is pathognomic finding in AA
Represents acute inflammation to the hair bulb in the anagen phase leading to the hair getting progressively thinned and weak
Get short hairs where the proximal end is narrower than distal end
Typically found at the edges of expanding patches and can be taken out with minimal traction
Black dots
Seen in acute alopecia areata
Represent hair shafts broken off at the level of the skin surface
Not exclusive to AA (also seen in trichotillomania, tinea capitis, dissecting cellulitis)
Yellow dots
Seen in chronic alopecia areata
Represent an empty hair follicle with a dilated ostium filled with scale and sebum
Again is not specific not specific to AA (also seen in androgenetic alopecia, chronic discoid lupus and dissecting cellulitus)
Groups of yellow dots typical of AA
Others:
Short vellus hairs
Broken hairs
Hair pull test:
Can be useful for confirming active hair loss
HISTOPATHOLOGY
Perform if have uncertainty about diagnosis
Take from edge of patch of active hair loss and include a few remaining hairs
Typically do x2 4mm punch biopsies that extend ito subcut fat on scalp
Ask for vertical sectioning of one and horizontal sectioning of the other
The picture you see depends on the stage of the disease
Acute disease:
Get an intense peribulbar, lymphocytic infiltrate surrounding the anagen follicle and the inflammation looks like a ‘swarm of bees’
May see follciluar oedema, cellular necrosis and pigment incontinence
Chronic disease:
Get folllicular miniaturisation
Essentially with time the hair follicles ‘get frightened’ and are converted to telogen hairs
(so see increased proportions of hair follicles in catagen or telogen phase)
By this stage the inflammatory infiltrate is usually less pronounced
May see pigment casts (clumps of melanin within the hair follicle)
You can then get either:
Recovery -reduced inflammation and regrowth of hair
or
Follicular scarring - with long standing inflammation can get permanent loss of hair follicles with only the sebaaceous gland left
ASSOCIATIONS
Nail Disease:
Seen in 10-20% of AA patients
Nail involvement has been associated with greater severity of disease
It can present in various ways:
Pitting commonest
Trachonychia (roughened nail plate)
Onychorrhexis (longtidudinnal fissuring nail plate)
Red spotting lunulae
Onycholysis
Onychomadesis (detachment of proximal nail plate from nail bed)
Autoimmune associations:
Vitiligo
Thyroid disease
Atopic dermatitis
Lupus erythematosus
Psoriasis
Genetic disorders associated with increased risk of AA:
Trisomy 21
Pyschosocial associations:
Increased mood and anxiety disorders
LAB INVESTIGATIONS
Often screen all adults and children for autoimmune thyroid disease
Thyroid disease can occur later so new symptoms should prompt checking thyroid tests again
Some propose only screening those at high risk for thyroid disease, ie those with:
Atopy
Trisomy 21
Family history of thyroid disease
Only need to test for other autoimmune diseases if have clinical symptoms or signs suggestive of them
PROGNOSIS
AA can persist for several years or indefinitely
Around 50% with limited patchy hair loss will recover within a year
Approx 66% patients show complete regrowth within 5 years
Unfortunately almost all will experience more than one episode of the disease (85-100% relapse rate over 10 years)
Regrowth sometimes begins with apperance of fine, white vellus hair
Approx 10% of patients with patchy disease progress to alopecia totalis or universalis
If get alopecia totalis - 75% will remain with AT
TREATMENT
Cosmetic options:
Can consider if patients don’t want traetment or who have incomplete responses
Wigs
Hairpieces
Sprays, lotions, powders to make hair appear more full
Eyebrow tattoing
False eyelashes
First line therapies:
Limited patchy (generally < 50% scalp affected)
Topical steroids
Intralesional steroids
Intralesional steorids:
Good therapy for adults with with isolated patches of hair loss
Generally people with < 25% hair loss are best candidates as hard to tolerate large number of injections
Beard/eybebrow: use concentrations of 2.5-5mg/ml triamcinolone in upper subcutis
Scalp: can use 5-10mg/ml triamcinolone in upper subcutis
New growth usually visible within 6-8 weeks
May repeat as required every 4-6 weeks and stop once regrowth is complete
If no response at 6 months should discontinue treatment
Method:
Inject 0.1ml or les into multiple sites 1cm apart
Dose per visit determined by extent of disease and toelrance but is usually 20mg or less on scalp
Don’t give more than 40mg per treatment
Adverse effects:
Skin atrophy (usually resolves within few months)
Hypopigmentation (be careful in pigmented skin particularly)
Telangiectasia
(Work in progress)
ANDROGENETIC ALOPECIA
(Also known as patterned hair loss: eg Female or Male pattern hair loss)
Essentially get a progressive non-scarring miniaturization of hair follicles located in characteristic areas of the scalp in genetically predisposed patients
Male:
Classically get a receding hair line and/or bald spot on vertex of scalp that progressively gets worse over time
Females:
Different than that in men
Get increased hair shedding, diffuse reduction in hair volume or both over the mid-frontal scalp
Women often notice they have less hair on the top of their head and may notice that the scalp is more visible than previously
The size of the ponytail becomes smaller in diameter so a good queestion to ask is:
“If you tie your hair back in a ponytail, how thick is the ponytail compared to 5 or 10 years ago or before you started losing you hair?”
FEMALE PATTERN HAIR LOSS
Most common form of alopecia in women
Prevalence in caucasians (lower in Asian population)
3-12% 3rd decade
14-28% 6th decade
29-56% women aged ≥ 70
PATHOGENESIS
Complex polygenetic aetiology involving several genes involved in androgen or oestrogen activity
Pathogenesis is not identical in male and female pattern hair loss
Androgen binding to hair follicle androgen receptors is important in the pathogenesis
Most women with FPHL have an increased sensitivity of the hair follicle to normal androgen levels (although sometimes FPHL can occur unrelated to androgen status)
Increased androgen sensitivity leads to miniaturization of hair follicles over time with progressive transition of terminal to vellus hairs
Hair is shorter, thinner and paler
Get diameter variability between different hairs
Women also get shortening of the anagen phase with premature catagen entry
All these factors lead to the phenotype of FPHL