INTRODUCTION
Malignant melanoma is the 3rd most common skin cancer and the most common cause of skin-cancer related death
Incidence is increasing
Lifetime risk:
3-5 per 100,000 Mediterranean
50 per 100,000 Australia/NZ
16 per 100,000 UK
Only 25-42% of melanomas arise in pre-existing naevi
Risk factors:
Environment:
History of sunburns and intermittent-high intensity sun exposure!!!
Artificial light (eg tanning beds)
Proximity to equator
Personal:
Fair skin, red hair, freckles, blue eyes
Increasing age (but is commonest cancer < 30 year olds)
Proximity to equator
Genetics:
The major susceptibility gene mutation associated with familial melanoma is CDKN2A
DNA repair defects (as in xeroderma pigmentosa) can also be implicated
Gene mutations seen in melanoma itself:
BRAF, CDKN2A, NRAS, TP53 gene mutation in cutnaeous melanoma
BRAF, NRAS, NF1, KIT in acral melanoma
SF3B1 in mucosal melanoma
Phenotypic expression of gene and environment interactions include:
Presence of naevi
Ephiledes (freckles)
MELANOMA SUB-TYPES
Superficial spreading (70%)
Is the most common sub-type
It is related to intermittent expsoure to sun and it is localised most often on the back of legs in women and on the back in men
Nodular melanoma (15-30%)
Most often occurs on the trunk and limbs of patients in 5th/6th decade of life
More common in males than females
Has a vertical growth phase, correlated with more rapid growth and higher rate of metastasis
Lentigo maligna melanoma (5-15%)
Unlike NMM and ssMM it correlates with long-term sun exposure and increasing age
It is mainly located on the head and neck
May evolve for decades before invading into the papillary dermis
Clinically it can show a variety of colours: black, brown, grey
It has irregular outlines and although the tumour is often relatively large and flat, a focus of invasion may be detected as a papule or nodule
Acral lentiginous melanoma (approx 5%)
This is uncomon
Accounts for 5% of melanoma in white people but it is the most common type in Asian, Hispanic and African patients
Amelanotic melanoma:
Malignant cells don’t produce melanin granules and lack pigment
Therefore ABCD criteria doesn’t really work
Treatment and prognosis tend to be similar to a melanoma of similar breslow depth but often times they are diagnosed at a late stage due to the lack of pigment clinically
Desmoplastic melanoma:
An uncommon subtype that accounts for about 4% of all melanomas
It arises from the dermis
It often occurs in chronically sun-damaged skin, especially the head and neck region of elderly individuals
It is characterized by proliferation of spindle cells and abundant fibrous stroma, giving it a firm, fibrotic feel.
It often lacks pigmentation, making it harder to diagnose.
It tends to have a more locally aggressive growth pattern but less likelihood of spreading to distant sites compared to other melanomas.
Treatment involves wide local excision
Mortality is lower than for other melanoma subtypes if detected early
Overall, desmoplastic melanoma has a better prognosis than other types of melanoma when diagnosed at the same stage.
PATHOLOGY TERMS
Breslow’s thickness:
Measurement from the surface of the epidermal granular layer to the point of maximum tumour thickness at right angles rounded to the nearest 0.1mm
For example:
Melanomas 0.75-0.84mm will be reported as 0.8mm
Extension down hair follicles not included
Prognosis closely related to thickness of the lesion
99% of melanoma in-situ patients will be cured with simple excision
Breslow thickness and 5-year survival:
(Statistics may change with advent of newer therapies for advanced disease)
In situ: 90-100%
< 1mm: 80-90%
1-2mm: 70-80%
2-4mm: 60-70%
>4mm: 50%
Clarke level:
I: confined to epidermis
II: Invasion papillary dermis
III: Invasion to junciton of papillary and reticular dermis
IV: Invasion to reticular dermis
V: Invasion to subcutaneous fat
Not really used in practice so much anymore but may have relevance
Ulceration:
Full thickness epidermal defect including loss of Stratum Corneum and basement membrane
Will see reactive changes like fibrin deposition and neovascularisation
Can see a mixed inflamamtory infiltrate containing neutrophils
Pagetoid spread:
Individual cell proliferation in the upper levels of the epidermis
Similar pattern of epidermal spread seen in Paget’s disease of nipple
Pagetoid spread seen as a major criterion for MMis
Mitotic index:
Is a measure of tumour activity/aggression
Mitotic index is no longer part of the AJCC 8th edition staging but is still likely to be a very important prognostic indicator so mitotic rate should be collected for all invasive melanomas
A mitotic index ≥ 2 is considered to be a negative prognostic factor
Lymphovascular invasion:
Lymphovascular invasion refers to the presence of melanoma cells within lymphatic and/or blood vessels in the tumor specimen
It is identified by finding tumour cells within spaces lined by endothelial cells
It is associated with an increased risk of recurrence, lymph node and distant metastasis, and decreased survival
For Malignant melanoma 0.8-1mm, sentinel lymph node biopsy (SLNB) should be considered for those with:
Ulceration
A mitotic index ≥2
Lymphovascular invasion
Regression:
When there is a variable decrease in melanoma cells and the presence of a host response
You see dermal fibrosis , an inflammatory infiltrate, melanophages, ectatic blood vessels, epidermal attenuation and/or apoptosis of keratinocytes or melanocytes
Significance poorly understood
In some instances may imply there may of been an initial thicker Breslow thickness with increased risk of lymphatic spread or in other instances it may indicate a better prognosis as the host immune response is reacting against the melanoma
Tumour infiltrating lymphocytes:
Local histopathological reflection of host’s immune response against cancer cells
Studies indicate TILs are a favourable factor in MM
MELANOMA DIAGNOSIS
Diagnosis should be based on full thickness excisional biopsy (2mm margins) where possible
This enable the pathologist to charachterise the melanoma and assign a breslow’s depth
The histology report should follow the 8th edition of the AJCC TNM classication and include:
Melanoma sub-type
Breslow thickness
Presence of ulceration
Clearance of surgical margins
Should also include (even though not in AJCC classification anymore)
Mitotic rate
Regression
Lymphovascular invasion
In rare situations melanomas may derive from dermal mealnocytes (eg from giant congenital naevi, malignant blue naevi, malignant spitzoid melanoma)
In these melanomas the prognostic relevance of tumour thickness and SLN invovlement is questionable
AJCC 8TH STAGING SYSTEM (2017)
Once a diagnosis is made then the melanoma should be staged
A good phone app called ‘melanoma tnm8’ that is easily downloadable from app stores is useful to calculate the stage of the melanoma
First you want to identify the TNM charachteristics and then it can be staged.
There will be a clinical stage and a pathological stage (essentially pathological stage gives more pathological information about the regional lymph nodes)
TUMOUR STATUS
Tis: Melanoma in situ
T0: No evidence of primary tumour found
TX: Thickness can’t be determined (eg curetted specimen)
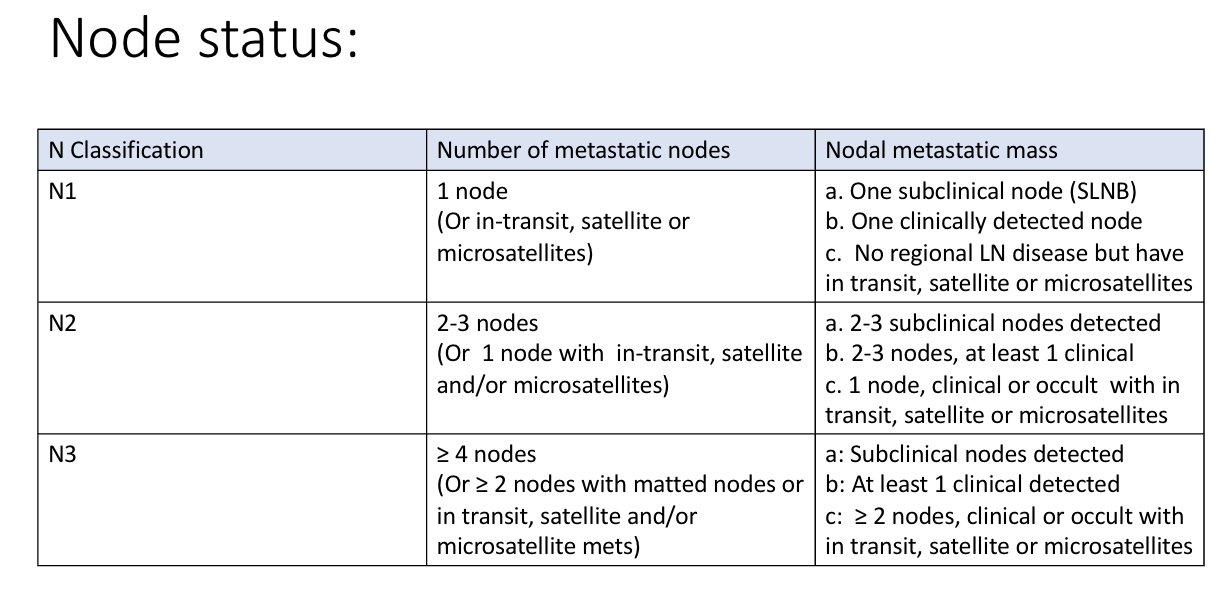
LYMPH NODE STATUS
Lymph nodes can be:
‘Clinically occult’ (detected by SLN biopsy)
‘Clinically evident’ (detected by clinical exam or imaging)
Microsatellites, satellites or in-transit metastases (MSI)
Microsatellite definition (AJCC 8th edition):
‘A microsatellite is a microscopic cutaneous and/or subcutaneous metasis adjacent or deep to, but discontinuous from, a primary melanoma detected on pathological exam of the primary tumour site.’
Satellite metastases mean clinically or pathologically detectable metastasis occurring within 2cm of primary melanoma
In-transit metastases mean the melanoma has spread to an area more than 2 cm away from the primary tumour but before the nearby lymph nodes.
a category seems to be reserved for sub-clincial nodes only
b category is assigned if nodes are detected clinically
c category is assigned if have microsatellites, satellite mets or in-transit mets (eg N1c, N2c or N3c)
DISTANT METASTASIS STATUS
CLINICAL STAGING
Staging can be divided into clinical staging and pathologic staging
Clinical staging includes micro-staging of the primary melanoma and clinical/radiologic evaluation for metastases
It should be used after complete excision of the primary melanoma with clinical assessment for regional and distant metastasis
Essentially:
Stage I-II is prior to any metastasis
Stage III is when MSI disease or regional lymph node involvement
Stage IV is when you have distant metastasis
PATHOLOGICAL STAGING
Pathologic staging includes microstaging of the primary melanoma and pathologic information about the regional lymph nodes
Once patholgoic staging is done can further divide Stage III from A to D by combining the T and N score
In the below chart you pick the N category on the left and the T category on top and pick the letter at the intersection
A=IIIA
B=IIIB
C=IIIC
D=IIID
MOLECULAR CHARACHTERISATION
BRAF analysis (NICE 2022)
Do not offer BRAF analysis from people with Stage IA or IB primary melanoma at presentation except as part of clinical trial
Consider for people with Stage IIA or IIB
Do BRAF if Stage IIC to IV
Other tests can be requested
Frequently mutated genes include:
BRAF, CDKN2A, NRAS, TP53 gene mutation in cutnaeous melanoma
BRAF, NRAS, NF1, KIT in acral melanoma
SF3B1 in mucosal melanoma
Other things that can be requested but whose clinical use is still limited:
PD-L1 expression (reported as the percentage of positive tumour cells for PD-L1)
Tumour mutational burden (expressed as a quantity of mutations in the tumour)
WIDE LOCAL EXCISION MARGINS
Managing stage 0 to II melanoma:
Clinical margin of at least 0.5mm MMis
1cm margin - stage 1 melanoma
2cm margin - stage 2
(Take into account primary melanoma margin)
STAGE 2 MANAGEMENT
Stage 2 is from pT2b (ie Breslow depth 1.1 with ulceration) to pT4b (ie Breslow depth > 4 with ulceration)
There is no lymph node involvement
These patients should have wide local excision to 2cm margins and sentinel lymph node biopsy
NICE guidelines state that you can consider whole body and brain imaging (usually contrast CT but MRI in some situations) with stage 2B melanoma and offer for patients with stage 2C and above melanomas
Patients with stage IIC tumours have worse prognosis compared to patients with other stage II tumours
Stage IIC has prognosis comparable to stage III tumours
NICE guidelines don’t suggest treating stage 2 melanoma with adjuvant therapy currently however there are ongoing studies regarding this cohort of patients
ASCO 2020 recommended that adjuvant immunotherapy or targeted therapy currently should not be offered to patients with resected stage II melanoma outside of enrolment in a clinical trial
Luke JJ et al, released results of a RCT comparing Pembrolizumab versus placebo as adjuvant ehrapy in completely resected stage IIB or IIC melanoma and reported that pembrolizumab may reduce the risk of recurrence in these groups by at least 35% so it will be interesting to see if the use of pembrolizumab or other immunotherapy becomes common practice in the future
STAGE 3 MANAGEMENT
Stage 3 management can be a bit more complicated
All patients with stage 3 disease should get molecular testing
They should also have a CT head and CAP (+ neck if head/neck/upper back primary)
I will talk a bit more about the following instances in the next few sections
Micro-satellite, satellite or in-transit disease without regional lymph nodes (N1c)
Clinically occult - Sentinel lymph node biopsy positive but not detectable on exam/imaging
Clinically evident - Cytologically/histologically proven lymph nodes picked up on clincial exam or through imaging
As mentioned before staging for stage III can be a bit confusing and sometimes counter-intuitive. Two points I would like to make are:
Stage 3A disease can be up to PT2a (ie up to 2cm breslow thickess w/o ulceration) with N1a/N2a category (up to 3 sub-clinical lymph nodes found on SLNB)
However if have micro-satellites, satellites or in-transit disease this upstages you right away to Stage 3B regardless if the lymph nodes are involved or not
MICRO-SATELLITE, SATELLITE, IN-TRANSIT DISEASE MANAGEMENT
NICE guidelines for MSI disease:
‘If have in-transit metastases offer surgery as the first option and if surgery is not feasible or has recurrent in -transit metastases consider one of the following options based on patient suitability’
Systemic anti-cancer therapy
Talimogene laherparepvec
Isolated limb infusion or perfusion
Radiotherapy
Electrochemotherapy
Topical treatment with imiquimod
Any of these more specialised options should be carefully weighed up against systemic treatmetns in order not to lower systemic treatment chances of providing long-term benefit
Some of these options have to be done in experienced centres
SENTINEL LYMPH NODE BIOPSY POSITIVITY
NICE guidelines criteria for SLNB:
Do not offer imaging or SLNB for stage 1a melanoma.
Consider SLNB for people with BT > 1
Consider SLNB for people who have melanoma with BT 0.8–1 and at least one of the following:
Ulceration
LVI
Mitotic index of 2 or more
If pregnant discuss delaying till SLNB till after pregnancy
Sentinel lymph node biopsy procedure:
The sentinel lymph node is defined as the first node in the lymphatic basin that drains the lesion and is the node at greatest risk for the development of metastasis
Biopsying this node can assist in staging patients at risk of metastatic disease
Procedure practical points:
Procedure takes about 30-45 minutes
Patient may go in day before procedure and radioactive dye (technicium 99) is injected at sites around the wound scar of where the primary melanoma was initially excised
On day of procedure they are given general anaesthetic
Once anaesthesised inject methylene blue also at the wound scar of the initial excision
A gamma probe is used which is then moved from the scar in the general direction of where the lymphatics would run
The gamma probe can sense the initial radioactive dye (technicium 99) and starts clicking more when you come across a ‘hot lymph node’
The site of highest radiotracer activity is identified
This area is then marked as the site of the planned biopsy site
A dissection is made into the subcutaneous tissue
The sentinel lymph nodes can be identified with both using the gamma probe to identify any ‘hot’ nodes and by also looking for any ‘blue’ nodes stained by the methylene blue
You then remove any sentinel nodes identified
If have negative SLNB this means the melanoma has likely not moved up there yet
It is important to do SLNB during the initial WLE as:
When doing the WLE you take a large amount of tissue around the primary site
This will cause alot of damage to the lymphatic tissue around the site so you will not get a true reflection of the lymphatic drainage in the area
The benefits of doing a SLN biopsy are that it can help to find out whether the cancer has spread to the lymph nodes
It gives prognostic information, for instance in people with primary melanoma between 1 and 4mm:
Around 1 in 10 die within 10 years is SLNB is negative
Around 3 in 10 die within 10 years if SLNB is positive
These numbers may improve now due to better systemic treatments
People who get a SLNB may be able to take part in certain clinical trials for new treatments but may be excluded if do not have this information
On the negative side of SLNBx there is actually no good evidence that people who have the operation live longer than people who do not have it
A general anaeshetic is required and operation complications can occur in 4-10 of every 100 people who have it
Also the results should be interpreted with caution - of every 100 who have a negative SLNB around 3 weill subsequently develop a recurrence in the same group of LNs
Previously if had a positive SLNB then patient would be sent for complete lymph node dissection but this is now not the case due to the findings of the MSLT-II trial
This was a large trial performed asking what is the value of complete lymph node dissection for patients with melanoma with sentinel-node metastasis?
The take home point from this trial was:
Completion lymph-node dissections at the time of the primary operation was found to improve local disease control as well as aiding in prognostication.
But there was no increase in survival from melanoma in patients who had complete lymph node dissection with sentinel node metastases compared to performing nodal observation with periodic ultrasounds of the sentinel node-positive basin.
It now appears widely accepted that routine complete lymph node dissection is not recommended following a positive sentinel lymph node
NICE guidance:
‘Do not routinely offer completion lymph node dissection with Stage III melanoma and micrometastatic nodal disease detected by SLNB unless:
There are factors that might make recurrent nodal disease difficult to manage and after discussion with the person and specialist skin cancer MDT, some examples mentioned in NICE guidance include:
Sitse where recurrent nodal disease would be difficult to manage (eg head/neck)
If Stage III adjuvant therapies are contraindicated
Regular follow up not possible
A MDT consensus meeting was convened in the UK in May 2018 to discuss the current role of SLNB in the management of cutaneous melanoma
They stated that emphasis should be placed on all patients at risk of metastatic disease since the primary role of sentinel lymph node biopsy is changing from that of a prognostic indicator to one that now influences access to adjuvant therapy
Complete lymph node dissection should not be recommended routinely for patients who have a postive sentinel node biopsy. Patients deemed at high risk should be considered for adjuvant therapy
A lymph node dissection shoul be considered for those patients who subsequently present with node ONLY recurrence having failed first line systemic treatment
CLND could also be considered for patients with features identified in their SNB that indicate a high risk of regional replapse:
Extra-capsular spread
≥ involved sentinel lymph nodes
Multi-focal or extensive disase (based on ‘Dewar criteria’)
AND who are unsuitable for adjuvant therapy (either due to medical co-morbidities or where geogrphical restraints may limit access to routine follow-up at regional cancer centre)
Following a positive SNB, patients who undergo observation rather than a CLND should have routine clinical exam and access to routine USS imaging of the nodal basin
CLND not recommended for primary head/neck melanomas with positive SLN where appropriate adjuvant therapy can be recommended but there is a concern that surgical rescue for this nodal basin is more complex than other basins and the potentially greater morbidity and expectation of post-op radiotherapy means these patients require very careful monitoring
Advantages and disadvantages of all treatment options should be discussed with the patient in these cases
Regarding adjuvant treatment in the case of a poistive sentinel lymph node here are some options (as per clinical management guideline for West of Scotland Network):
If have IIIA disease (ie only one node detected on SLNB) and the met is ≤ 1mm can consider observation
But if it is Stage IIIA with >1mm met or Stage IIIB/C should consider adjuvant systemic treatment
CLINICALLY EVIDENT LYMPH NODES (EXAM/IMAGING)
These are lymph nodes detected during clinical exam or by imaging and which have cytological or histological proof of melanoma often after sampling by fine needle aspirate or open biopsy
NICE 2022 guidance:
‘Offer therapeutic lymph node dissection to people with palpable IIIB to IIID melanoma, or cytologically or histologically confirmed nodal disease detected by imaging’
‘Consider systemic adjuvant treatments’
ESMO 2019
‘Detailed imaging should be performed prior to LN dissection (eg CT or MRI) to exclude distant metastases. Evidence of distant metastatic spread precludes surgery and qualifies patient for systemic therapy’
In the case of unresectable lymph nodes should consider systemic adjuvant treatment
ADJUVANT TREATMENT STAGE 3 RESECTED MELANOMA
ASCO 2020:
‘For patients with resected stage IIIA/B/C/D disease that is BRAF wild type the following options should be offered (no particular order):
Nivolumab x 52 weeks
Or
Pembrolizumab x 52 weeks
Ipilumamb and interferon are not recommended
‘For patients with resected stage IIIA/B/C/D BRAF-mutant disease (V600 E/K), the follwoing therapy should be offered (no particular order):
Nivolumab x 52 weeks
Or
Pembrolizumab x 52 weeks
Or
Dabrafenib plus Trametinib x 52 weeks
Caveat:
Patients with stage III disease with microscopic sentinal nodal mets < 1mm in diameter have not been included in trials assesing immunotherapy
They tend to have a relatively better prognosis and lower risk of relapse than stage III disease
Treatment should be individualised in these patients after discussing risk-benefits with these patients
MANAGING STAGE III UNRESECTABLE AND STAGE IV MELANOMA
Overall immunotherapies more effective than targeted therapies
Nivolomab plus ipilimumab most clinically effective (also cost effective)
But combination increases risk of toxcitiy so monotherapy could be considered for people with poor performance status/co-morbidities who maybe would not tolerate toxicity
Nivolumab and pembrolizumab have similar clinical effectiveness and cost effectiveness when used as monotherapies so can use either
(Ipilimumab not recommended as 1st line monotherapy)
If immunotherapy unsuitable than targeted therapies based on BRAF are an option:
Examples:
If have symptomatic brain mets needing steroids
If have high diseae burden/rapid progression and don’t have enough time to genereate immune response
Combination therapy best:
Trametinib + dabrafenib or Encorafenib + Binimetinib Have similiar effectiveness
If combination unsuitable monotherapy with dabrafenib or vemurafenib should be offered
If immunotherapy unsuitable and targeted treatment unsuitable/patient BRAF-wild type options are limited to chemotherapy with dacarbazine or best supportive care
Follow up Stage I, II, III melanoma:
Large retrospective studies show that between 60 and 80% of first recurrences are local and/or nodal
Annual risk of recurrence for tumours < 1.5mm: <6% first five years, under 1% next five years
Annual risk of recurrence for tumours > 1.5mm: higher risk of recurrence in first year, drops to < 2% after year five
Most studies indicate about 80% of recurrences occur within the first 3 years but unfortunately 16% of first recurrences have been reported to occur after five years and late recurrence (> 10 years) can occur
Increased mitotic rate has been found to be associated with risk of recurrence
Follow up:
Patients who had melanoma in situ don’t need regular follow up - they should be given information and education on personal regular skin surveillance and nodal disease
Surveillance imaging:
Routine surveillance should not be offered for patients with Stage I-IIB melanoma
Decisions on use of routine surveillane imaging for patients with Stage IIC-III melanoma should be made at regional MDT level
NICE benefits and disadvantages of surveillance imagin:
Advantages:
If melanoma recurs it is more likely to be detected sooner
This could lead to a better outcome by allowing treatment with drugs (eg immunotherapy) earlier
Regular scans may be reassuring to patient
Disadvantages:
Although early drug treatment of recurrent melanoma may improve survival, there is currently no evidence showing this
Regular scans may induce anxiety
Scans expose body to radiation, can increase risk of cancer
Scans of chest cause small increase risk of thyroid cancer
Scans may show abnormalities that are later found to be harmless causing unecessary investigations and anxiery
Management of advanced (unresectable Stage IIIC or IV) melanoma:
All patients with advanced melanoma should be tested for mutations in BRAF and have their management discussed at a specialist MDT to determine optimal management strategy
All patients with advanced melanoma should be offered the opportunity to participate in clinical trials
Metastasectomy:
May be option with distant skin, node and visceral inovlvement
Surgery of single or localised metastasis has been shown to be associated with improved survival
Kinase inhibitors:
The MAP kinase pathway in the end leads to the transcription of genes involved in cell proliferation and cell survival
When out of control the MAPK pathway can lead to cancer
RAF, MEK and ERK are all kinases
Kinases are enzymes which can transfer a phosphate group
Pathway:
Growth factors bind to tyrosine kinase receptors
This leads to transphosphorylation of the receptor and then a docking protein can bind to it (Grb2/SoS)
This activates RAS
4. RAS then activates a phosphorylation cascade (ie each protein phosphorylates the next protein downstream)
RAS activates RAF
RAF activates MEK
MEK activates ERK
5. ERK then enters the nucleus to activate transcription factors to promote transcription of genes which promotes cell survival and cell proliferation
Relevance to melanoma:
40% of melanomas have a mutated B-RAF protein kinase (V600E or K)
B-RAF mutations occur mainly in younger patients, and in torso melanomas and are associated with a poorer prognosis
B-RAF inhibitors:
Vemurafenib
Dabrafenib
Rapid disease remission is seen but drug resistance secondary to MAP-K reactivation or pathway bypass happens
Side effects:
• Fatigue
• Acne
• Photosensitivity
• New AKs, MM, SCCs (as body can get beyond pathways)
• Increased LFT
• Arthtralgia
• Nausea
• Alopecia
• Dry skin (particulalry palms and soles)
• Secondary malignancies
Vemurafenib vs dabrafenib:
Vemurafenib:
More photosensitive
Poorer response against brain metastatis
Dabrafenib:
20% more effective
Better against brain metastasis
More pyrexia
More palmo-plantar hyperkeratosis
Trametinib:
Oral MEK inhibitor
4-month average survival by itself
MEK-induced paronychia is a side effect
Trametinib can cause a decrease in left ventricular systolic function so ECG and ECHOs are often performed as a baseline and during treatment
Combinations of BRAFi and MEKi
The combination of a BRAF inhibitor and a MEKi enhances survival of the drug compared to using either treatment alone (13.4 months average)
MEK-induced paronychia is a side effect
Trametinib can cause a decrease in left ventricular systolic function so ECG and ECHOs are often performed as a baseline and during treatment
Combination of BRAFi + MEKi vs BRAFi alone
Response rate combo 64-68% vs BRAFi alone 45-51%
Progression free survival combo 9.3-11.4 months versus BRAFi alone 6.2 to 8.8 months
Toxicity profile for grade 3-4 side effects: 34-65% combo versus 28-63% BRAFi alone
Trametinib in combination with dabrafenib is recommended for patients with unresectable Stage IIIC or Stage IV melanoma with a BRAF V600 mutation
Imatinib:
C-kit mutation inhibitor
Mucosal and elderly/chronic sun-exposed skin melanomas (10-20%)
Small response with monotherapy
Immune checkpoint blockers:
CTLA-4 (cytotoxic T-cell antigen) inhibitors (eg ipilimumab)
CTLA-4 is essentially an inhibitory receptor that inhibits T-cell activation
Two signals are required for T cell activation:
Peptide MHC and CD80/86 on the antigen presenting cell have to bind with the T cell receptor (TCR) and CD28 on the T cell respectively
CTLA-4 can bind to CD80/86 (the same receptor CD28 binds to)
This results in an inhibitory signal and T cell activation is inhibited
Inhibiting CTLA-4 allows for unregulated T cell activation and these T cells can then be used to fight melanoma
Ipilimumab is a fully human monoclonal antibody against CTLA-4:
Give four IV infusions over 12 weeks
Get a slow but steady decline in tumour burden
Two year survival is 24% (x 2 of placebo group)
Autoimmune side effects:
Dermatitis
Colitis
Hepatitis
Uveitis
Neuropathy
Endocrine dysfunction
Approximately 2% death rate but early steroid intervention helps a lot
Clinical trials currently ongoing for resected stage 3 melanoma
PD-1 and PD-1 ligand:
Melanoma carries a protein called PD-1 ligand (PD-L1)
This acts ‘like a mask’ making the T cell think the melanoma cell is a healthy cell
PD-1 on approaching T effector cells bind to PD-L1 on the melanoma cell
They think it is a healthy cell and don’t mount an immune response
Therefore blocking PD-1 on the T cell (eg pembroliuzmab, nivolumab) or PD-1 ligand allows the Teff cell to recognise the melanoma and effect a response against it
PD-1 monoclonal antibodies:
Pembrolizumab 2mg/kg 3 weekly until remission
Nivolumab 3mg/kg 2 weekly until remission
Relatively similar efficacy and side-effect profile
Side effects generally related to revving up of the immune system and get autoimmune involvement affecting pretty much any organ:
Fatigue and a maculopapular rash is common
Autoimmune thyroid disease
Colitis
Adrenal suppression
Hepatitis
PD-1 associated potential cutaneous reactions:
Erythroderma or morbilliform rash on starting
Pruritus with a maculopapular rash (most common)
Pruritus without rash
Vitiligo/leukoderma - can involve retina, common and associated with better prognostic outcome
Psoriasis
Eczema
Bullous pemphigoid eruption
Lichenoid eruption
Granulomatous (sarcoid-like) reaction
TEN/SJS/EM
Lupus erythematosus
Alopecia (AA and telogen effluvium)
Grade 3-4 toxcicity rates are generally lower with single agent nivolumab (11.7%) and pembrolizumab (10.1-13.3%), higher with ipiliumamb (10-19.9%) and highest with the combination of nivolumab and ipilimumab (55%)
Overall survival at 5 years in comparative melanoma study (NEJM 2019, Larkin et al):
Nivolumab and Ipilumumab: 52%
Nivolumab monotherapy: 44%
Ipilumumab monotherapy: 26%
Current NICE guidelines:
BRAF V600 positive:
Vemurafenib
Dabrafenib +/- trametinib
BRAF negative or failed:
Ipilimumab and Nivolumab combined
Pembrolizumab monotherapy
Current oncology practice (regardless of NICE):
Only use BRAFi to rapidly debulk a large potentially fatal tumour load if BRAF +ve or if failed a checkpoint inhibitor
Young/middle age patients should use Ipilumumab and Nivolumab combination
Older patients use Pembrolizumab monotherapy
Stop CPIs after 5 years if under remisison or if severe side-effects (underreported as patients prefer to stay on drug)
Systemic agents are standard treatment for stage 4
Stage IIC options:
Consider Keystone trial enrolement with PD-1 therapy
Stage 3 options:
• Stage IIIa or b: observe or adjuvant PD-1 therapy
• Stage IIIc or d: adjuvant PD-1 therapy
3rd checkpoint inhibitors coming out: ????? Unsure name : ? Retalizumab (? CAG-3 blocker)
T cells get exhuasted even if overstimulated
These CIs keep that T cell going so when combined with PD-1 inhibitor again success rate is similar
Isolated limb perfusion:
Surgical technique allowing loalised delivery of high dose of chemotherapy (usually melphalan)
It is a significant surgical undertaking and should only be performed in specialist centres
Previously considered in 2 clinical situations:
Adjuvant treatment for high-risk primary melanoma
In general not recommended in this instance
Therapeutic treatment for major limb recurrence of melanoma
Is a treatment option for patients with bulky disease confined to one limb
Ablative therapies:
CO2 laser ablation:
Short wavelength energy in focused light beam to destroy tumour nodules
It can be applied under local anaesthetic and can be repeated
Can be considered for multiple lesions on the trunk or abdomen or for limb disease
Electrochemotherapy:
Short electric pulses to increase absorption of intralesional or IV chemo
Can be used inpatients who have had prior surgery, radiotherapy and isolated limb perfusion
Can be considered after MDT team discussion and after careful consideration of alternative systemic therapy options, or when other options exhausted
Bone mets
Consider radiotherapy
Spinal cord compression:
Will need urgent referral to a surgeon
No clear evidence to support or refute the use of radiotherapy (in combination with other treatments) to alleviate pain and neurological deficit associated with spinal cord compression
Brain mets:
Common finding at autopsy but diagnosed in only approximately 10% of patients before death
Options:
Corticosteroids
Resection
Radiotherapy
Radiosurgery (stereotactic radiotherapy) - can be considered in inoperable disease
Systemic treatment
Extras to consider:
Surgery margins special circumstances:
Modifications with reduced safety margins may be acceptable for preservation and fucntion in acral and facial melanomas and may be carried out with Slow Mohs
In lentigo maligna where it may be difficult to complete full WLE margins radiotherapy can be considered
Occasionally in LM 0.5mm are not adquate as still can get atypical cells beyond visible edge - could consider MOHs. Other options include radiotherapy and topical imiquimod but caution should be applied when doing this
Talimogene laherparepvec (T-VEC)
Is an oncolytic virus therapy approved in 2015 for treating melanoma.
It is a genetically modified herpes simplex virus that selectively replicates in and kills cancer cells while sparing normal cells.
T-VEC is injected directly into melanoma tumors, where it causes tumor cell lysis and death and stimulates an anti-tumor immune response.
In clinical trials, T-VEC improved durable response rates compared to chemotherapy or immunotherapy alone in advanced melanoma patients.
It is the first oncolytic virus therapy to be FDA-approved.
Isolated limb infusion (ILI) and isolated limb perfusion (ILP) are regional chemotherapy techniques used to treat melanoma confined to an arm or leg.
During ILI/ILP, the blood flow of the limb is isolated from the rest of the body.
High doses of chemotherapy agents, typically melphalan with or without actinomycin-D, are circulated through the limb while avoiding systemic exposure.
This allows for delivery of localized high-dose chemotherapy directly to the melanoma tumors in the limb.
ILI/ILP has been shown to achieve high response rates in patients with advanced melanoma confined to a limb, including complete responses in some cases.
It can be used in patients who are not candidates for surgery.
While associated with some local toxicity, ILI/ILP largely avoids the systemic side effects of chemotherapy.
It provides an alternative limb-sparing treatment option for locally advanced melanoma.
Electrochemotherapy is a localized treatment that uses electric pulses to enhance the uptake of chemotherapy drugs like bleomycin or cisplatin into tumor cells. It is performed by first administering the chemotherapy drug intravenously. After a short time to allow drug distribution, small electric pulses are applied directly to the tumor using electrodes. This transiently permeabilizes the tumor cell membranes allowing for increased drug uptake.
Electrochemotherapy has demonstrated high objective response rates for the local treatment of metastatic melanoma nodules on the skin or nodal disease. It can be used to control tumor growth and symptoms when surgical removal is not possible. The procedure is well-tolerated with minimal side effects. While not a first-line treatment, electrochemotherapy provides a targeted approach to achieve local control of melanoma tumors that may be resistant to other therapies. Its ease of use makes it a feasible option for localized melanoma control.
Special circumstances:
Do full staging with MRI instead of CT in people < 24 and pregnant women with Stage IIB to IV
Consider MRI instead of CT if have mitotic index ≥ 5 or primary melanoma scalp
SIGN:
PET-CT does appear to have higher sensitivity and specificity for detection of mets but the quality of evidence is not great.
Consider PET-CT in patients with indeterminate findings on CT or for patients who are being considered for major surgical resection
Lab investigations:
Elevated LDH in the absence of clinical symptoms or signs is the first indicator of stage IV disease in 12.5% of patients
LDH is now included in AJCC classification system in patient’s with advanced disease
Evidence and availability for tumour markers (S10 protein, melnaoma inhibitory activity (MIA) protein and tyrosinase mRNA) are limited and not routinely indicated
Adjuvant radiotherapy:
Should we do adjuvant radiotherapy after complete LN dissection in high risk for recurrence patients:
Phase 3 trial showed it reduced risk of relapse in the irradiation field by 50% but had no impact on recurrence free survival and overall survival
ESMO recommendation: Overall as local control is rarely the therapeutic objective in melanoma, adjuvant RT is no longer routinely recommended but can be discussed in specific cases where local control is critical (eg head and neck melanoma)
Other considerations for adjuvant radiotherapy in melanoma:
Inadequate resection margins LMM
After some resections of melanoma metastases
After resection of bulky disease